
Synthetic Tissue Engineering
Tissue engineering is a field where engineering & biology are used "to restore, repair or regenerate the functions of tissues and organs" (Petrella & Spaggiari, 2018). Synthetic tissue offers an alternative to typical grafts of human or porcine (pig) parts, as well as an alternative to traditional organ donation. Basically, researchers take or make a scaffold out of structural proteins, inject it with stem cell/stem cell precursors, and then put the scaffold into a simulating environment so that they grow into their proper functions.
In research for synthetic tissue “...conventional methods of cell culture are often performed in a 2D environment, consequently limiting the tissue structure to the planar form, which is very different from the complex 3D structure in organisms” (Hsu & Chen, 2018). This is why, for complex organs and advanced tissue engineering, scaffolding is so important - because especially in materials such as hydrogel, the cells can grow in a more humanistic replication of what would be created within the body (Kobel & Lutof, 2011).

Above: "A decellularized human heart awaits rebuilding with an injection of precursor cells (Ott Lab 2013).
Lungs
The lungs are the human body's way of disposing of carbon dioxide and taking in oxygen, a critical part of maintaining the blood's pH and providing much needed resources to cells throughout the body. With a rise of problems like COPD (chronic obstructive pulmonary disease), tobacco smoking, air pollutants, vaping, and respiratory failure-related illnesses such as COVID-19, the need for transplantable lungs ever growing (Petrella & Spaggiari 2018). However, there is a widespread shortage of organs and chronic rejection of organs that are transplanted. Tissue engineering lungs could pose a solution to the organ shortage and perhaps an alternative to typical organs, that require long-term immunosuppression.
Artificial lungs are incredibly complex to tissue engineer, as many specialized lung tissues are required for proper functioning (Petrella & Spaggiari 2018). Therefore, while experimental transplantation has been successful, the transplantable/tissue-engineered lung is very far away. In the ongoing research, a lot of work is being done to see how tissue-engineered lungs could become a reality. Importantly, tissue engineers have moved away from the 2D model to the 3D in order to be able to get the many functions of specialized lungs correct.
In order to build a 3D lung, most researchers start by obtaining a lung scaffold, either natural or artificial. Typically, a natural lung scaffold might be something like a xenogenic scaffold from porcine tissue (pig lungs), which is already commonly used in heart valves & vascular stents. Artificial scaffolds are also under development, with some possible candidates in synthetic hydrogels like PEG (polyethylene glycol) and PVA (polyvinyl alcohol) and synthetic elastomers like PGS (poly glycerol sebacate). No matter the scaffold, the material must be non-immunogenic, biocompatible, non-toxic, chemically stable, and well tolerated by the host (Petrella & Spaggiari 2018).
Once the researchers have obtained a lung scaffold, they typically inject it with the stem cell precursor stells, as is typical for most tissue engineering. As previously mentioned, lungs have many types of cells with specialized purposes. For example, lungs contain epithelial cells, which line the airway lumen, endothelial cells, which line the pulmonary vessels, smooth muscles cells, alveolar macrophages, and the lung's own resident stem cells that do self-renewal (Petrella & Spaggiari 2018). Researchers then attempt to get the cells to grow in the scaffolding and take up their duties by simulating the environment/movement of the lung, which spurs the cells to action. The goal is to make organoids, or highly developed 3D models which are made from many stem cells (single stem-cell lungs don't currently exist).
Brendan Maher of Nature explains the challenges of making tissue for complex organs such as the lungs:
“Researchers have had some success with growing and transplanting hollow, relatively simple organs such as tracheas and bladders. But growing solid organs such as kidneys or lungs means getting dozens of cell types into exactly the right positions, and simultaneously growing complete networks of blood vessels to keep them alive. The new organs must be sterile, able to grow if the patient is young, and at least nominally able to repair themselves. Most importantly, they have to work — ideally, for a lifetime" (Maher 2013).
Beyond the research on scaffolding materials, decellularization, proper injection (recellularization), and simulating the lung environment, much more is being done. Researchers are looking into...
-
the lung's self-renewal response; lungs have a lot of stem cells in them already (type II alveolar epithelial cells are progenitors, or stem cell precursors). Researchers have been exploring how they can get lungs to do the recellularization process of the scaffold themselves.
-
air-liquid interface cultures - using the patient's own airway epithelial cells to reduce the risk of organ rejection.
-
direct scaffold reseeding using autologous stem cells (adult cells), trying to figure out how to get adult mature cells to behave like embryonic stem cells.
-
modeling of typical cell damage caused by problems like cystic fibrosis, asthma, and tobacco-induced damage (Petrella & Spaggiari 2018).
-
developing lung models, such as the "lung-on-a-chip" model, which may be used in the more near future as respiratory assist systems or oxygenators, also possibly as models for lung diseases (Petrella & Spaggiari 2018; Daly 2017).
Heart
In many ways, tissue engineering hearts is very akin to tissue engineering lungs; like lungs, hearts are very complex organs with many types of cells and structures, requiring 3D models and scaffolding in order to develop properly.
In the next phase, the ECM is "recellularized;" researchers inject some combination of cells back into the scaffolded heart. These injected cells are generally stem cells or precursor cells (pre-stem cell cells) derived from embryonic cells, iPS cells (induced pluripotent stem cells), or mature adult cells (Maher 2013). Embryonic cells are cells derived from 3-5 day embryos; they are effectively stem cells that are able to grown into many types of cells (in other words, they are pluripotent). iPS cells are adult cells that are reprogrammed to take on embryonic properties. Based on their type and injection location, the injected cells will take on different functions. For example, endothelial cells (for lining blood vessels) are often made using iPS cells (Maher 2013).
After these cells are injected into the ECM, the matrix is termed "colonized" and put into a bioreactor. This bioreactor/chamber mimics the heart's environment, mechanical loads, and electrical signals. The mimicry helps the cells to go down the correct developmental path, growing by sensing the factors of their environment. Cells are actually quite adaptive, they just need time, proper nutrients, and the correct conditions (Maher 2013).
maAs it is, like lungs, the technology of bioengineering hearts isn't quite where it needs to be to meet the high demand for hearts. Because of the complex demands on hearts and the nature/ethics of the organ, research on transplanting bio-engineered hearts hasn't yet gone past transplanting rat hearts (Maher 2013). It is already complicated enough to transplant new aortic valves (such as porcine valves)... new synthetic materials and whole hearts have a whole host of as of yet largely unmet
requirements. For example, repairing materials must blend well with the rest of the heart, as collagenic scarring can lead to reduced functioning (Zhao et al. 2020, pp. 593-616). Heart bioengineering material must also be incredibly resistance to cyclic failure, the material smooth, elastic, non-immunogenic, non-toxic, biocompatible, and durable. There is no room for error here. And as of 2013, while researchers were able to get hearts to beat, none were able to get them to pump blood (Maher 2013). For the time being, more research must be done.
Currently, hearts are made through a process of decellularization, recellularization, and stimulation. First, in the decellularization phase, tissue engineers take pig hearts or old human hearts, and start by pumping a detergent through the networks of blood vessels and aorta. This detergent strips the hart of its bloods, fats, DNAs, proteins, sugars, and other cellular material, so that the new organ will be accepted by its new host's immune system (Maher 2013). What is left behind is called a "scaffold heart," also known as an "extracellular matrix," or ECM, made up of structural proteins like collagen and various laminins. This ECM is the bare-bones structure for the heart that serves to make the new heart grow properly and look like a heart.
Above: "Harold Ott and his collaborators test the durability of bioengineered heart parts;" a heart is decellularized and grafted. (Nature 2013).

Above: A visualization of the decellularization, recellularization, and stimulation process for a bioengineered heart. (Spencer, N., 2013).
Skin
Skin matrices are important outside of the body as they not only provide protection for the internal organs and muscles but also act as a barrier toward foreign objects. Skin also withstands the most direct contact and mechanical forces, therefore tissue must be highly strong and elastic. Skin’s protection is mostly provided via the maintained homeostasis of the skin’s microenvironments (Zeng et al. 2011) and the regeneration of skin cells which allow for a fresh barrier to be created every time the skin sheds old cells. Regeneration of natural skin begins with epidermal cells at the top of the skin moving to the site of the injury and re-epithelialization occurring and then for the skin to recreate the “basement membrane” of the skin which houses base layers such as the dermis to the epidermis (Zeng et al. 2011).
-
Grade 2: mild rejection; 11 - 50% of vessels have infiltrates of small lymphocytes, variable eosinophils or mild spongiosis; no epidermal or stromal infiltrates or large lymphocytes; basal vacuolar change
-
Grade 3: moderate rejection: 51%+ of vessels have infiltrates of small lymphocytes, variable epidermal and stromal inflammation, at most mild spongiosis, possible endothelial plumping, eosinophils and large lymphocytes; fusion of basilar vacuoles to form clefts and microvesicles
-
Grade 4: severe rejection; 51% of vessels have infiltrates of small lymphocytes, but also dyskeratosis, epidermis has heavier lymphocytic infiltrates and moderate to severe spongiosis; stroma shows infiltrates extending into base of epidermis; also endothelial plumping, eosinophils and large lymphocytes; separation of epidermis from dermis" (Hamodat, 2011-2020).
Bioengineered skin is a promising opportunity for long-term skin tissue regeneration:
“Even though skin tissue engineering is still a relatively young discipline, it has no choice but to mature in order to be translated into clinical applications. Systems based on bioengineered skin... offer several advantages. These comprise the ability to grow cells that retain their functionality, including stemness, needed for long-term tissue regeneration. This bioengineered skin not only provides a clinically relevant alternative in the field of skin regeneration but has also led to the development of a robust preclinical platform for human pathologies in which innovative strategies such as gene and cell therapies can be evaluated.”(Guerrero-Aspizua et al., 2018).
In most injury cases, this process is uninhibited and works well at creating viable skin. Some injuries which create an environment impossible for natural skin growth are a large wound where the base growth of skin becomes damaged, high degree burns, conditions such as diabetes and bullous condition, and surgical interventions. In most cases, skin grafting would be used to close a wound and allow for the patient to heal, however, full-thickness skin wounds which include the removal of the base layer of skin cannot exceed 4 cm. in diameter for this method of skin healing to work effectively (Herndon et al., 1989). Skin grafting also has its own risks with there being possibilities of either donor sites becoming infected or the rejection of the grafted skin onto the area of injury.
Below is a scaling for the rejection of skin grafting which is typically decided on 8 weeks after implantation:
"Proposed grading system for rejection of full-thickness cadaver skin transplant for large abdominal defects:
-
Grade 0: no rejection; no perivascular infiltrates; normal skin
-
Grade 1: indeterminate for rejection; 1 - 10% of vessels have infiltrates of small lymphocytes; no eosinophils, large lymphocytes, spongiosis, epidermis or stromal inflammation; basal vacuolar change
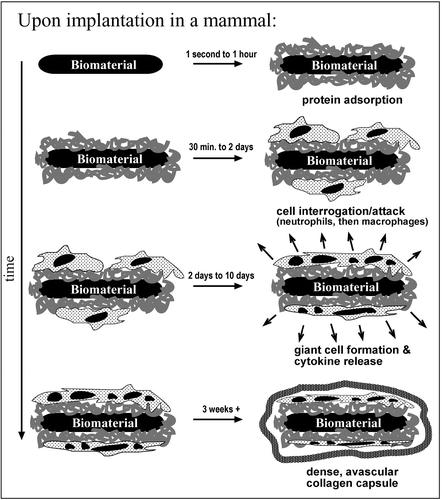
Above: "Different reactions toward biomaterials being inserted into a mammal" depicted over the term of three weeks (Ratner and Bryant 2004).
These issues with the grafting of existing “natural” skin onto areas of injuries have led to much progression in the area of tissue-engineered skin. A silicon semi-permeable membrane used to be used as a valid substitution for skin grown meanwhile the base layer of skin would be able to reproduce, however, research has lead to biopolymers as the way forward with skin grafting. The engineered skin is typically more biocompatible with less probability of rejection and with similarity in both macrostructure and performance to “natural” skin (Zeng et al., 2011). Protein-based materials and polysaccharide-based materials are usually used in these skin tissues as a base material for the engineered tissue. Collagen
Type I has been used as a base layer due to its triple-helical structure which allows for it to be a prime candidate in the scaffolding of the base dermis
Above: "Professor Robert Brown, Head of UCL's Tissue Repair and Engineering Center, discusses research on new ways to engineer tissue" depicted with collagen and how to make skin using this material (UCL 2010).
layer of the skin. Collagen-based materials have been beneficial in creating capillary pathways and have been shown to both generate dermis and epidermis for the injury site (Still et al., 2003). Fibronectin has also been used as a base layer for skin tissue creation as it has a lot of benefits with the way it allows for blood clogging and overall creation of semi-permeable barriers for the area of injury (Zeng et al., 2011).
Bioengineered Applications (Lungs, Heart, Skin) Comparison:
The environmental/material requirements for tissue-engineered lungs, hearts, and skins are quite varied.
Lungs are complicated with tissue engineering and its many components of alveoli, stem cells, and the outer muscle of the lungs. The main aim of lung tissue engineering is to provide an alternate solution to treat lung disease as it affects more than 11 million people in the United States alone (Akter et al., 2016).
Lungs are usually engineered using scaffolding with the need that the scaffolding is able to remain stable, sanitary, biocompatible, and stable for cells to multiply. However, some of the scaffolding can not be the best to replicate the lung due to inadequate mechanical properties or degrade at different rates. In order for scaffolding to be optimal, it must be created out of multiple materials in order to fully simulate a natural lung. Some research has been done with using extracellular cell matrices to scaffold lung tissue as it may be a better option than the traditional mix of materials to make scaffolding (Akter et al., 2016).
_edited.jpg)
Above: Necessities of what a tissue engineered lung needs to have in order to function well inside of the human body (Akter et al., 2019).

Above: The process of how a genetically engineered heart is created from the "cardiac tissue engineering approach" (Zhao et al., 2020).
The heart is one of the most complex organs in the body with a physiological pump that cardiomyocytes (CMs) take up 80-90% of heart volume which support multiple other types of cells such as endothelial cells and fibroblasts. The engineered heart must have the ability to not only keep the volume needed in order to oxygenate and pump blood but also to be able to withstand many spontaneous pumps and keep up the contractions (Zhao et al., 2020) needed to sustain homeostasis within the body.
One of the ways to create a 3D model of the heart is through using scaffolding of either collagen, fibrin, or Matrigel which allows for the model to be as large or as small needed for the patient. Scaffolding is usually used for heart engineering due to its high usability and applications. The other version of heart engineering centers around a cell method sheet where the material is stacked on top of each other creating a 3D model of the
heart which bonds itself together with confluent sheets of CMs and whatever the extracellular matrix produces (Zhao et al., 2020). Either way, the heart’s base is usually created one of these two ways with different ways to engineer tissue into the spaces where the scaffolding is empty. This can either be done through directed cardiac differentiation, electrical stimulation, and patch assembly with some of the ways to be completed (Zhao et al., 2020).
Skin is typically engineered layer by layer since it is composed of different types of skin. A new advancement in the skin is cultured epithelial autografts (CEAs) which are cells sheets use to cover large injuries, typically found in burn victims for the epidermal layer (Stojic et al., 2019). The epidermal layer alone has some drawbacks because it does not allow for a base to fully grow along with the injury in question. Dermal substitutes such as a layer of keratinocytes or autologous fibroblasts are used as a dermal connective layer. These substitutes, however, are very costly to produce and might need additional surgery in order to function properly (Stojic et al., 2019).
With the addition of other scaffolding, bioprinting is also used to create skin parts to the engineered skin. Typically the layers are composed of a mix of collagen, fibroblasts, and keratinocytes in order to make a skin similar to the skin found naturally. Bioprinting is typically used either in laser or extrusion-based with the main material usually composed of collagen (Stojic et al., 2019).
Beyond differences in creating the tissues, there are also different environmental requirements for the heart, lungs, and skin. A few differences: first, the heart and lungs have a more narrow allowable pH range, compared to the skin, which experiences more environmental conditions. The blood must maintain a pH range of 7.35-7.45 or risk acidosis or alkalosis, which leads to a quick death (Surat 2018). While the lungs are less sensitive to pH change than the heart (they regulate pH change by obtaining oxygen and releasing carbon dioxide), they still hover around a neutral pH ("Lungs," 2015; "What's a Normal Blood pH," 2019). By comparison, the skin hovers in the acidic range, of about 4.7 - 5.75 ("Skin's pH," n.d.), and has more direct contact with acidic and alkaline materials. Second, the heart and lungs experience less direct contact with foreign objects, requiring less resilience to varied climates, pHs, and mechanical loads. So while the skin has to be able to withstand a wider variety of stresses (and thus must be both tough and elastic), the mechanical loads are usually sharper and for lower time durations. By comparison, the heart withstands some mechanical stress caused on it by surrounding organs, normal growth, and its function (its ventricles and atria must expand and contract), however it most of the load is distributed (Tomoike 1996). During normal development, your heart adapts to its mechanical load; in fact, the heart’s shape and circulation properties are determined by mechanical load endured during development (Tomoike 1996; Arts et al 2005). Finally, the skin withstands direct radiation, UV exposure, and a wider range of temperatures, the lungs and heart do not have the same problems. Overall, although the skin might seem less complex to grow, as it can more often be grown with 2D engineering (compared to the complex structures required for the lungs and heart), the materials have more requirements to meet as its environmental conditions are more demanding.
Some Types of Tissue Engineering Materials
Researchers have engineered many, many types of tissue, but for the most part, they stick to polymers, multi-polymer composites, and polymer ceramic composites. Polymers and best match up with the human body's composition; polymers are carbon-based repeated structures, just like the three key components of human life: carbohydrates, lipids, and proteins.
Carbohydrates are the simplest of the three, small chains of carbon, hydrogen, and oxygen (C, H, O). Lipids are much more complex, longer carbon chains, made up of fatty acids (and also composited of carbon, hydrogen, and oxygen). Proteins are the most complicated of the three, made up of carbon, hydrogen, oxygen, nitrogen, and sulfur. Proteins form the structural and globular (repairing, growing, maintaining) components of the human body, like muscle tissue, hemoglobin, insulin, antibodies, enzymes, keratin (in our nails and hair), and collagen, a connective matrix that forms organ scaffolding/structure ("Carbohydrates, Lipids, and Proteins", n.d.).
For this reason, polymers best match existing tissue. Both natural and synthetic polymers are used in tissue engineering, with natural polymers having better responses with tissues, and synthetic polymers having more tunable properties (Brahatheeswaran et al., 2011). Of polymers, typically, thermosetting polymers (like PLA, PGA, and PCL) cannot achieve the requirements of biomaterials for things like heart muscles, which require softness, elasticity, and resistance to long-term cyclic strain. Instead, elastomers are better, as they have superior material properties with respect to elasticity (Chen et al., 2007).
Ceramics (bio-ceramics) have been used for decades in medical device engineering, replacement and repair of various tissues, and as interfaces between hard and soft tissues, as they have been shown to have better tissue responses than both polymers and metals (Hench 1998). Bio-ceramics are common in bio-inert, permanent devices like hip replacements and bone reconstruction (Hench 1998); however, new resorbable bio-ceramics (whose metal components are easily processed by the metabolism) show huge promise for soft tissue applications (Baino et al., 2015).
Comparatively, metals are usually a poor choice for tissue engineering; they inflame nearby tissue, cause allergic reactions, corrode, have trouble withstanding biological fluids (non-biocompatible), and are not, for the most part, biodegradable. Also, their materials properties, specifically low elastic modulus, make them a poor choice for most synthetic tissues (excepting, perhaps, joints), which require stretching and easy integration with existing tissue (Prasad et al., 2017).
Some of the research on polymeric scaffolding materials is summarized below. In short, many many materials are used in tissue engineering:
Below: "List of commercial polymeric scaffolds’ products," (Brahatheeswaram et al., 2011, Table 2)

